European Medicines Agency en LinkedIn: EMA recommends approval of Bimervax as a COVID-19 booster vaccine -…

European Commission - Vaccinate, vaccinate and boost! Evidence gathered by the European Medicines Agency and the European Centre for Disease Prevention and Control (ECDC) suggest that mRNA vaccines used as boosters 3
Third COVID-19 dose approved by EMA 28 days after second vaccination - Hospital Pharmacy EuropeHospital Pharmacy Europe

Brianda Echevarria on LinkedIn: EMA evaluating data on booster dose of COVID-19 Vaccine Janssen - European…

Repeat booster shots have immune-system risks: European Medicines Agency | World News - Hindustan Times
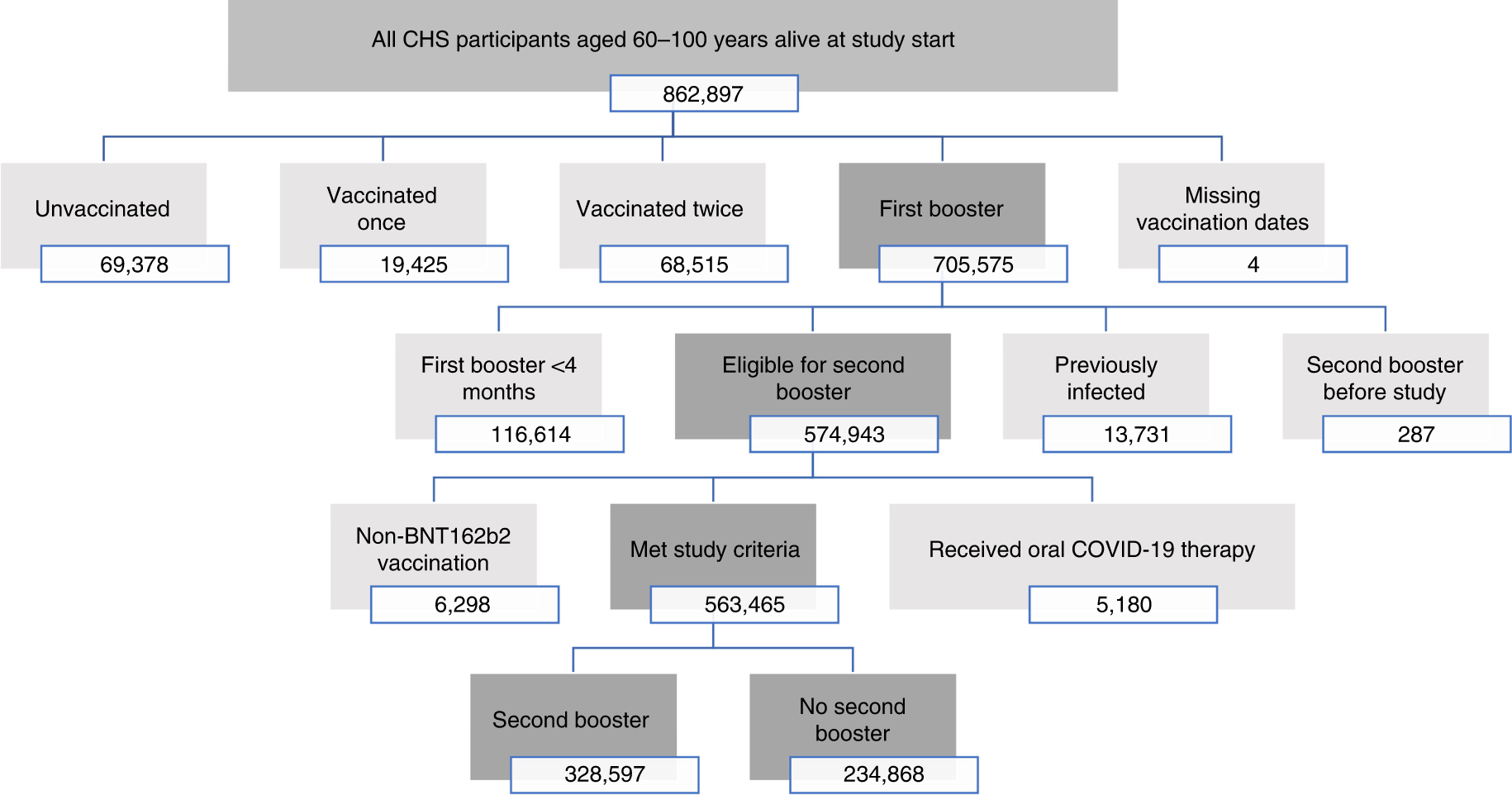
Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years | Nature Medicine
ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines | CDE Almería - Centro de Documentación Europea - Universidad de Almería

European Medicines Agency Recommends Second COVID Booster For People Over 60 - As WHO Ponders Status Of COVID Emergency - Health Policy Watch